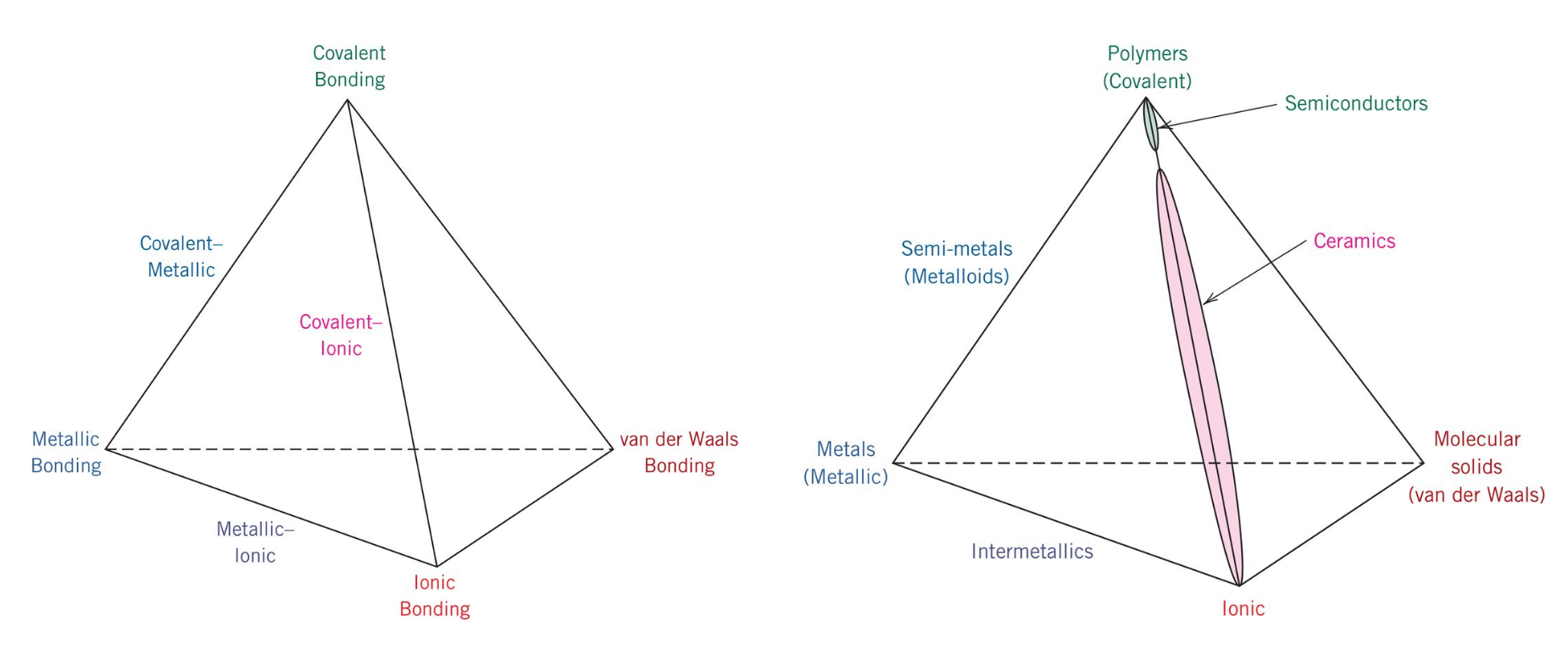
Between a metallic and non metallic element the bond will be different than it would be between two metals and two non metals.
What type s of bonding would be expected for rubber.
Ionic bonding metallic bonding covalent bonding with some van der waals bond van der waals bonding.
I would look up covalent and ionic bonding.
What type s of bonding would be expected for each of the following materials.
Before attempting rubber bonding it is advisable to carry out a solvent degrease.
Problem 4feqp from chapter 2.
What type s of bonding would be expected for each of the following materials.
Expert answer 100 13 ratings.
For rubber the bonding is covalent with some van der waals bonding.
Rubber is composed primarily of carbon and hydrogen atoms.
Acetone may be too aggressive as certain types of rubber are vulnerable to attack.
A ionic bonding b metallic bonding c covalent bonding with some van der waals bonding d van der waals bonding solution the correct answer is c.
What type s of bonding would be expected for each of the following materials.
What type s of bonding would be expected for rubber a ion.
What type s of bonding would be expected for each of the following materials.
Also it should be noted that the bond types vary between different types of elements.
Surfaces may have mold release slip additives or other processing lubricants on them so a quick wipe with isopropanol is a good idea.
Solid xenon calcium fluoride caf2 bronze cadmium telluride cdte rubber and tungsten.
2 4fe what type s of bonding would be expected for rubber.
What type s of bonding would be expected for rubber.
Brass a copper zinc alloy rubber barium sulfide bas solid xenon.
Materials science and engineering 9th edition edit edition.
Also upon this same tangent the bond between two atoms of the same element will be a different type of bond than that which.